The latest data released by the Chinese Center for Disease Control and Prevention shows that influenza viruses are the main detected pathogens recently. The overall influenza epidemic is at a high prevalence level, with the positive rate of influenza virus detection showing an upward trend, though the growth rate is slowing down.
Influenza is an acute respiratory infectious disease caused by influenza viruses, characterized by rapid transmission and easy mutation. It is classified as a Class C statutory infectious disease in China. Facing such a "variable" opponent, timely vaccination remains one of the most effective ways to prevent infection and reduce the risk of severe illness.
Figure 1 Common Influenza Vaccines
1. Overview of Influenza Viruses
The influenza virus is like a sophisticated "microscopic fortress," consisting of three layers from the outside to the inside: the envelope, matrix protein, and core (containing RNA and nucleoprotein). Among them, the two most notable glycoproteins on the envelope surface are hemagglutinin (HA) and neuraminidase (NA) (Figure 2). You can think of HA as the "smart key" for the virus to invade cells, specifically identifying and opening the doors of host cells; while NA acts like a "molecular scissors," responsible for "cutting" newly assembled viruses out of the cell to continue infecting the next target.
Precisely because these two proteins are so crucial in the infection process, the design of influenza vaccines is based on this principle: allowing the immune system to safely "recognize" these proteins in advance. Whether it is traditional inactivated/attenuated vaccines or modern recombinant protein vaccines, their essence is to present HA and/or NA to the human body in a harmless form, equivalent to providing the immune system with a "wanted notice of virus characteristics." In this way, when the real virus attacks, the body can quickly produce antibodies to accurately intercept it, achieving effective protection.
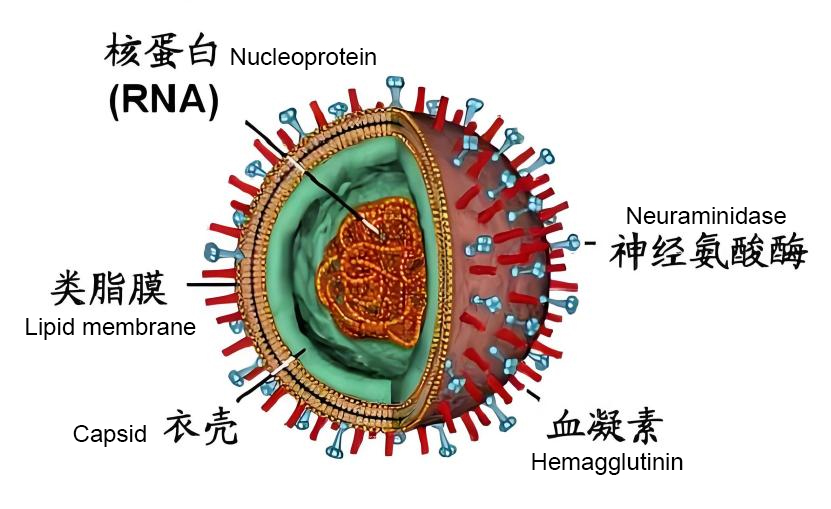
Figure 2 Schematic Diagram of Influenza Virus
2. Difficulties in Influenza Vaccine Purification
The purification process of influenza vaccines faces multiple technical challenges: its core antigens (such as hemagglutinin HA and neuraminidase NA) have characteristics of high glycosylation modification and conformational sensitivity, making them prone to structural denaturation or functional inactivation during purification; in addition, the virus particles themselves are wrapped by a double-layer lipid envelope, with low physicochemical stability and extreme sensitivity to changes in parameters such as temperature, pH value, and shear force; at the same time, the process system needs to accurately separate the target antigen from the complex virus lysate and completely remove potential sensitizing impurities such as ovalbumin, host cell proteins, and residual DNA.
Against this background, the purification process must strike a balance between impurity removal efficiency and protection of antigen structural integrity, which directly affects the immunogenicity and batch-to-batch consistency of the vaccine. Currently, mainstream processes on the market mostly adopt a 1-2 step chromatography combination strategy, including gel filtration chromatography based on size exclusion principle, ion exchange chromatography utilizing charge differences, and mixed-mode chromatography based on hydrophobic interactions. In recent years, with the application of automated control systems and the development of new fillers, the synergistic effect of multi-step chromatography has been significantly improved, providing a reliable guarantee for vaccine quality.
3. Influenza Vaccine Purification Processes
3.1 Chicken Embryo Influenza Vaccine Purification
Chicken embryo (egg-based) vaccines are the mainstream technology for large-scale production of influenza vaccines. This technology involves inoculating recombinant influenza viruses into developing chicken embryos for amplification, then harvesting the allantoic fluid containing the viruses, followed by further separation and purification of the viruses.
One of the main challenges of this process is the high concentration of residual ovalbumin, which can account for more than 60% of the total protein content in the harvested fluid and is an important potential factor causing allergic reactions. Traditional purification methods, such as depth filtration and density gradient centrifugation, have limited efficiency in removing such impurities and are difficult to balance the efficiency and stability requirements of large-scale production.
To address this, modern industrial-scale purification usually adopts a multi-step integration strategy: first, preliminary clarification and impurity removal are performed through centrifugation and microfiltration/depth filtration, then concentration and buffer exchange are carried out by ultrafiltration, and finally, high-precision purification of virus particles is achieved through chromatography technologies (such as molecular sieves, ion exchange, or mixed-mode chromatography). This comprehensive process not only increases production capacity but also significantly reduces residual impurities such as ovalbumin, ensuring vaccine safety.
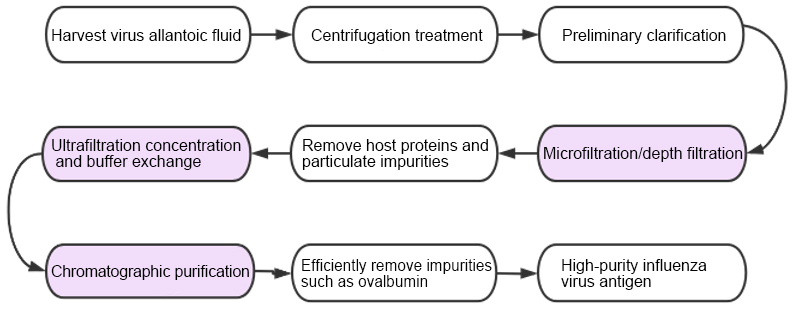
Figure 3 Chicken Embryo Influenza Vaccine Purification Process
3.2 Cell-Based Influenza Vaccine Purification Process
Compared with traditional chicken embryo-derived vaccines, influenza vaccines produced based on mammalian cell culture technology have an important advantage: their production process completely avoids the use of chicken embryos, thus fundamentally eliminating residual ovalbumin and significantly reducing the risk of allergies related to vaccine vaccination.
The process first performs deep clarification of the harvested cell culture fluid to remove cell debris and insoluble impurities. To further improve product purity and safety, specific nucleases are usually added in the post-clarification step to efficiently degrade residual DNA from host cells. The purification stage mostly adopts mild and efficient low-pressure chromatography technologies (such as molecular sieves, ion exchange, or mixed-mode chromatography), achieving highly selective separation and purification of virus particles while maintaining the structural integrity of viral antigens. This technical route not only ensures vaccine safety but also enhances the controllability and consistency of the production process.
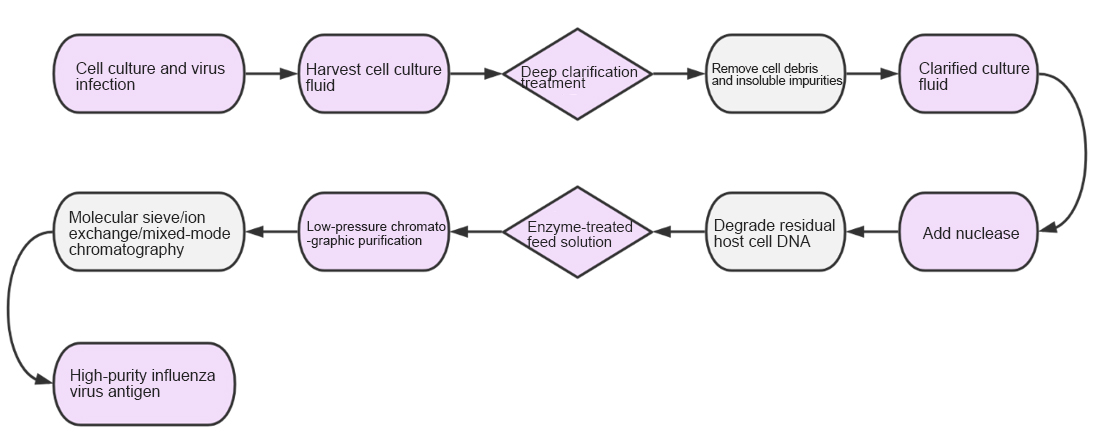
Figure 4 Cell-Based Influenza Vaccine Purification Process
4. Hanbon Technology's Influenza Vaccine Production Solutions
Large-scale vaccine production is a systematic project with high requirements for process stability, purification efficiency, and production capacity controllability. Among them, the downstream purification link is the core of the entire production chain, directly determining the purity level, safety standards, and production economic benefits of the final product. Any fluctuation or inefficiency in the purification process may affect the consistency, immunogenicity, and market supply capacity of the vaccine.
To address the complex challenges of this link, Hanbon Technology provides a full-cycle integrated solution for downstream purification. Based on the in-depth integration of processes and equipment, we offer customized, integrated, and modular production units for customers, and rely on automated control systems to achieve precise control and full traceability of process parameters. This system aims to help vaccine manufacturers improve product recovery rate, enhance batch-to-batch consistency, and shorten the process cycle, thereby accelerating the industrialization process of vaccines and ensuring the stable supply of high-quality vaccines while meeting regulatory requirements.

Figure 5 Bio-Pro Process Chromatography System & ACC Automatic Axial Compression Chromatography Column

Figure 6 Bio-TFF Automatic Tangential Flow Filtration System & Bio-Con Inline Conditioning System
5. Conclusion
From the perspective of purity and safety, the purification of influenza vaccines is a key quality control point in their production process. Whether based on chicken embryo or cell culture process routes, the core goal of purification is to completely remove process-related impurities such as host proteins and nucleic acids while maximizing the maintenance of the structural integrity and immunogenicity of viral antigens. Currently, the continuous development and integration of downstream purification technologies such as chromatography and ultrafiltration have significantly improved vaccine purification efficiency and batch consistency, not only ensuring the safety of the final product but also laying a technical foundation for maintaining stable protective efficacy in large-scale vaccinations.