According to Eli Lilly and Company's 2025 Q3 financial report, its metabolic therapy drug tirzepatide (including the anti-diabetic version Mounjaro and the weight-loss version Zepbound) achieved quarterly sales exceeding $10.1 billion, with cumulative sales of $24.837 billion in the first three quarters. It officially surpassed Merck's pembrolizumab (Keytruda) to become the new global "Drug King."
This transition came at least a year earlier than market expectations, marking not only the commercial success of a single drug but also signaling a profound shift in the global pharmaceutical industry's focus. For the first time, the metabolic disease treatment sector has topped the global pharmaceutical sales rankings, breaking the long-standing dominance of oncology drugs.
About Tirzepatide
Tirzepatide is a dual receptor agonist of GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1). It targets the root cause of obesity to reduce appetite and food intake. Previously, tirzepatide has been approved by the NMPA for:
Adult patients with type 2 diabetes whose blood glucose remains inadequately controlled with metformin and/or sulfonylureas despite diet and exercise;
Long-term weight management in adults with an initial body mass index (BMI) meeting the following criteria, on the basis of diet control and increased physical activity: ≥28 kg/m² (obesity), or ≥24 kg/m² (overweight) accompanied by at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, hyperglycemia, obstructive sleep apnea, cardiovascular diseases, etc.).
Tirzepatide consists of 39 amino acids from 17 types (including 37 encoded amino acids and 2 non-encoded amino acids). It contains a side chain similar to that in semaglutide, which is a dimer of C20 fatty diacid linked via glutamic acid and 2-(2-(2-aminoethoxy)ethoxy)acetic acid, attached to the lysine residue of the side chain.
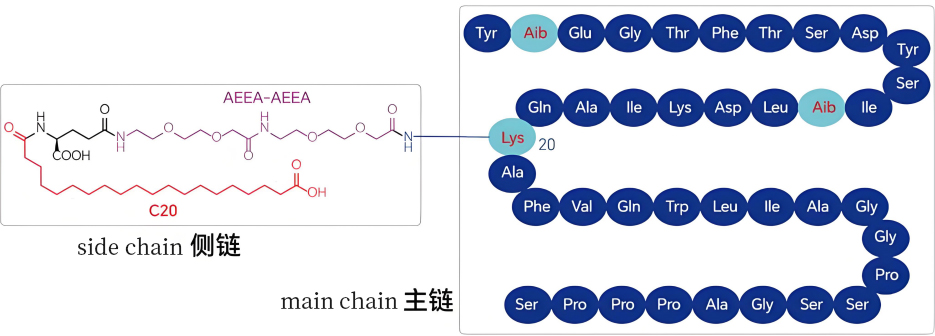
Schematic diagram of tirzepatide structure
Unlike semaglutide, which incorporates only one unnatural amino acid (Aib) in its polypeptide backbone, tirzepatide introduces two unnatural amino acids in the main chain. This significantly increases the difficulty of synthesizing tirzepatide via biological fermentation, leading current industrial processes to rely primarily on solid-phase synthesis or a combination of solid-liquid synthesis methods.
Production Processes
Currently, over 100 peptide drugs have been launched globally, with more than 200 undergoing clinical trials. These drugs are widely used in multiple medical fields such as immunology, oncology, and cardiovascular diseases. Among them, GLP-1 class drugs (for obesity and type 2 diabetes treatment) and growth hormones stand out as market leaders, demonstrating the enormous potential and future development trends of the peptide drug sector.
For the production of GLP-1 class drugs, biological fermentation and chemical synthesis are the primary methods for manufacturing GLP-1 receptor agonists.
Biological Fermentation:
Biological fermentation is a method that uses microorganisms (e.g., bacteria, yeast, or mammalian cells) to produce drugs. In preparing GLP-1 receptor agonists, genetic engineering techniques can insert genes encoding GLP-1 analogs into host cells, which are then cultured to express the target peptide. The peptide is subsequently extracted from the cells and purified to obtain the final product.
Advantages of biological fermentation:
(1)Capable of producing peptides with structures similar to endogenous GLP-1 in the human body;
(2)Through selective expression of specific genes, GLP-1 analogs with improved properties (e.g., longer half-life or enhanced biological activity) can be produced;
(3)Biological systems can perform post-translational modifications, which are crucial for the stability and biological activity of peptide drugs.
Chemical Synthesis
The upstream of chemical synthesis mainly adopts solid-phase or liquid-phase peptide synthesis, i.e., amino acid coupling to synthesize peptides via resin carriers or in liquid solution systems. This includes the use of Solid-Phase Peptide Synthesis (SPPS) technology, where amino acids are added one by one to the growing peptide chain.
Advantages of chemical synthesis:
(1)Precise control over peptide sequence and modifications, enabling highly customized drug design;
(2)Rapid synthesis of large quantities of peptides, suitable for large-scale production;
(3)Introduction of unnatural amino acids or chemical modifications during synthesis to improve drug stability, solubility, or bioavailability.
Traditional peptide drug development primarily relied on biological fermentation, which suffered from high production costs, low purity, and poor stability. With advances in peptide synthesis technology, modern peptide drug development mainly depends on artificial synthesis. Most currently marketed peptide drugs are prepared via chemical synthesis, with solid-phase synthesis dominating due to its high crude product yield, high purity, short development cycle, and the ability to split synthesis into multiple fragments outsourced to different manufacturers, effectively mitigating risks.
Production Challenges
As a GIP/GLP-1 dual receptor agonist, tirzepatide's molecular design incorporates two unnatural amino acids (D-Ala and N-Me-Ile), distinguishing it significantly from semaglutide, which contains only one unnatural amino acid (Aib). This difference directly increases the complexity of its production process, particularly posing multiple technical bottlenecks for biological fermentation and forcing current industrial production to rely primarily on chemical synthesis.
1. Limitations of Biological Fermentation
Biological fermentation synthesizes target peptides via recombinant microorganisms (e.g., Escherichia coli or CHO cells), but it has inherent limitations in compatibility with unnatural amino acids:
Genetic Coding Restrictions: Natural amino acids are encoded by 20 standard codons, while unnatural amino acids require artificial modification of the host cell's genetic system (e.g., introduction of orthogonal tRNA/aminoacyl-tRNA synthetase pairs). Tirzepatide requires simultaneous expression of two unnatural amino acids, placing extremely high demands on strain engineering; in contrast, semaglutide only requires Aib insertion, making it more compatible with fermentation.
Translation Efficiency and Product Purity:Incorporation of unnatural amino acids reduces host cell translation efficiency and may cause abnormal peptide folding or incorrect ligation. For example, D-Ala insertion requires chiral inversion via enzymatic kinetic control, while N-Me-Ile methylation relies on additional methyltransferases. These steps extend the process cycle and increase impurity risks (e.g., unmodified natural amino acid fragments).
Economics of Large-Scale Production: Fermentation relies on high-yield cell factories, but tirzepatide's complex structure makes stable scale-up difficult via traditional processes. As a linear peptide of 39 amino acids, even with a 99.5% coupling efficiency per step in solid-phase synthesis, the theoretical yield of the full-length sequence drops sharply. Minor flaws in any step accumulate and amplify in the final product.
2. Challenges of Chemical Synthesis Pathways
Currently, industrial production of tirzepatide primarily adopts a hybrid strategy combining Solid-Phase Peptide Synthesis (SPPS) and Liquid-Phase Peptide Synthesis (LPPS), splitting the peptide into key fragments for efficient ligation to balance yield and purity. However, this pathway still faces core challenges:
Fragment Selection and Ligation Efficiency: The 39-amino-acid tirzepatide must be split into 4–5 fragments (e.g., AA1–14, AA15–21). Short fragments improve single-fragment purity (>99%) but increase LPPS ligation steps (each with ~70–80% yield), leading to a drastic drop in overall yield; long fragments may reduce purity due to folding difficulties or increased side reactions.
C-Terminal Epimerization Risk: During liquid-phase ligation, the amide bond at the C-terminus of fragments is prone to α-stereocenter epimerization (especially in Fmoc-protected systems). For example, the C-terminal fragment of tirzepatide (AA30–39) contains D-Ala, requiring strict control of temperature (<5°C), reaction time (4–6 hours), and use of high-efficiency coupling reagents such as HATU to reduce isomer formation.
D-Ala Introduction:Chiral inversion of D-Ala typically uses Sharpless asymmetric epoxidation or enzymatic kinetic resolution, requiring additional protective groups (e.g., Boc or Fmoc) and deprotection before fragment ligation, increasing process steps and impurity control complexity.
N-Me-Ile Synthesis:Methylation requires introducing methylating agents (e.g., methyl iodide or dimethyl sulfate), but their strong alkaline conditions may disrupt peptide backbone amide bonds, causing deamidation byproducts. Thus, methylation is usually performed late in fragment synthesis under mild conditions (e.g., low temperature, limited reagent dosage).
Tirzepatide's two unnatural amino acid design, while conferring superior efficacy, has pushed its production process to new heights. This represents not just an increase in steps but a fundamental shift from the relatively "biologically friendly" fermentation model to a high-difficulty, high-control "precision chemical synthesis" model. In the future, semi-synthesis (fermentation + chemical modification) and continuous flow synthesis technologies are expected to further optimize tirzepatide's production efficiency and quality consistency, laying the foundation for the widespread application of dual-target peptide drugs.
Purification Case Studies
Tirzepatide synthesis primarily adopts a combined SPPS-LPPS strategy to balance efficiency and purity.
1. Combined Solid-Phase/Liquid-Phase Synthesis
Eli Lilly's tirzepatide is synthesized by producing multiple short peptide fragments (AA1-14, AA15-21, AA22-29, and AA30-39) via SPPS, followed by ligation of these four fragments via LPPS to obtain the final product. In the SPPS of these short fragments, amino acids are not coupled individually; instead, Fmoc- or Boc-protected dipeptides or tetrapeptides are used for coupling to form longer fragments. This reduces amino acid usage, minimizes impurity generation, and improves production efficiency and product quality.
2. Solid-Phase Synthesis
Rink amide resin is used as the solid-phase carrier for stepwise amino acid coupling. For example, Fmoc-Ser(tBu)-OH is first immobilized, followed by repeated deprotection (piperidine/DMF) and coupling (HOBt/DIC-activated carboxyl groups) until the target sequence is complete.
After synthesis, the resin is cleaved with trifluoroacetic acid (TFA) to remove side-chain protecting groups, yielding the crude peptide.
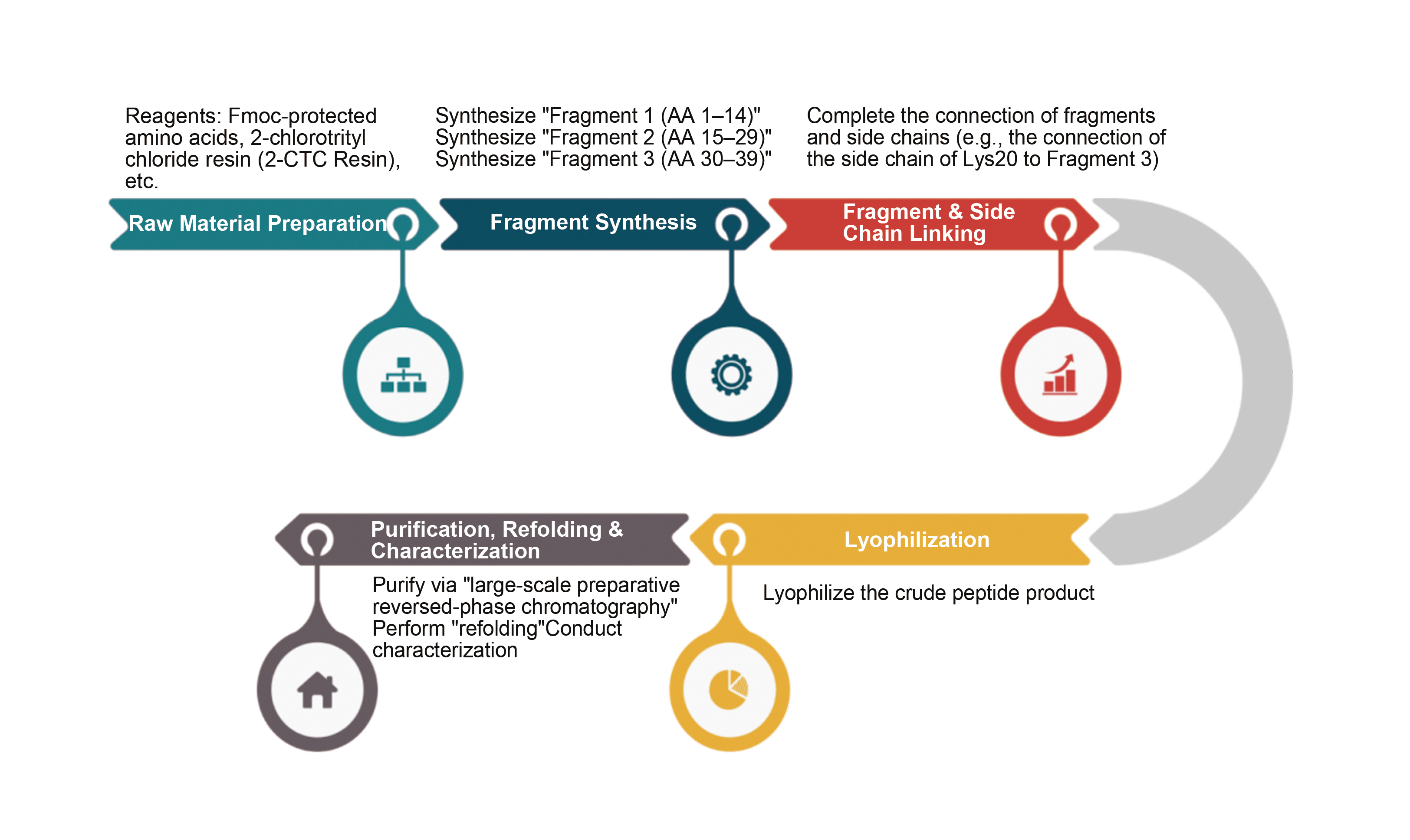
Tirzepatide Manufacturing Process Flow
Impurities associated with peptide synthesis are mainly classified into four categories:
1.Process-related impurities introduced during synthesis: missing peptides, truncated peptides, inserted peptides, unprotected peptides, and other peptide-related substances;
2.Degradation impurities: formed via deamidation, oxidation, reduction, hydrolysis, disulfide bond mispairing, β-elimination, or other instability factors;
3.Polymers: dimers and multimers;
4.Optical impurities: racemization and diastereoisomer impurities.
These impurities can be controlled or removed during synthesis or purification. Factors influencing peptide purification and their optimization strategies are summarized below:
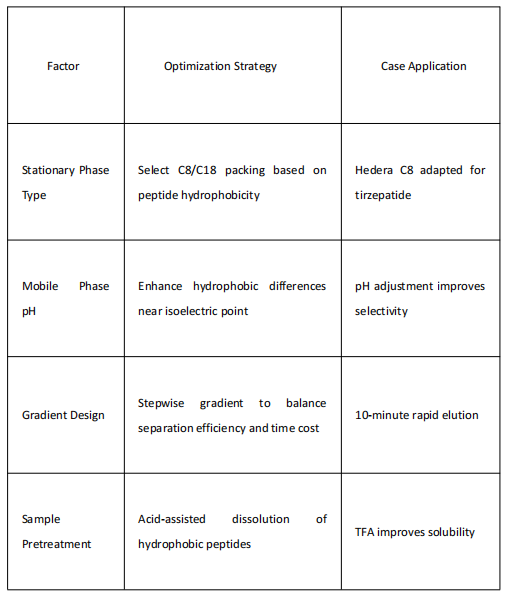
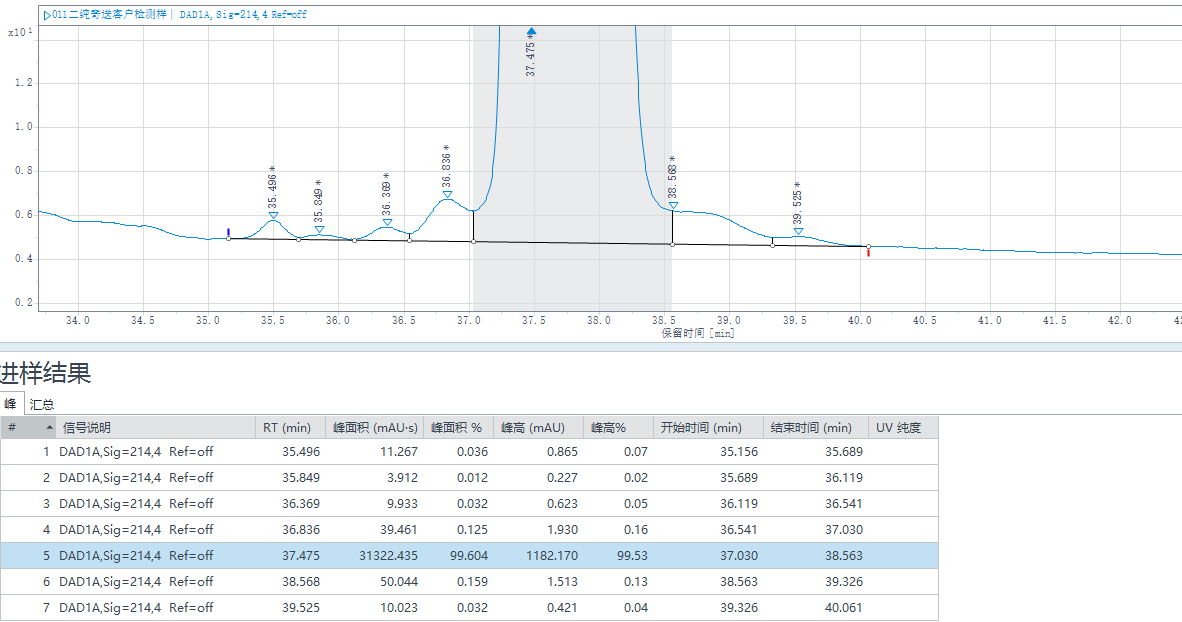
The Application Technology Team of Jiangsu Hanbon Science & Technology Co., Ltd. successfully purified tirzepatide samples to over 99% purity via two-step high-pressure purification, addressing the complex impurity profile (including uncoupled fragments, epimers, deamidation products, etc.).
Hanbon Science & Technology Tirzepatide Lab-Scale Purification Data
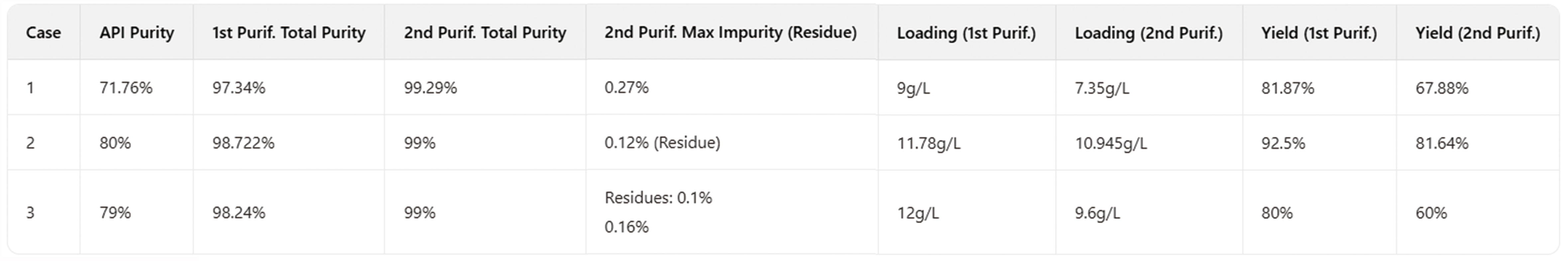
Purification Case 2 Details
Column: 10 × 250 mm
Packing: Hedera C8
Sample Loading: 235.64 mg (as-purified)
Elution Method:
1st Purification Chromatogram
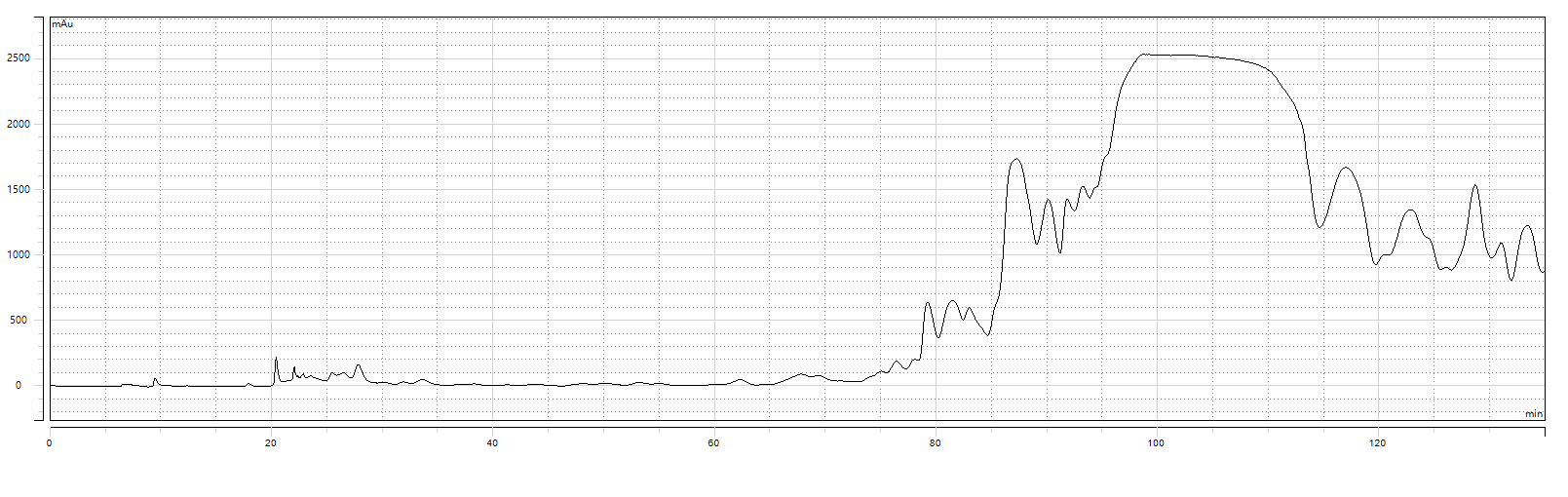
1st Purification Test Results:Purity: 98.722%;Yield: 92.90%
.png)
2nd Purification Chromatogram
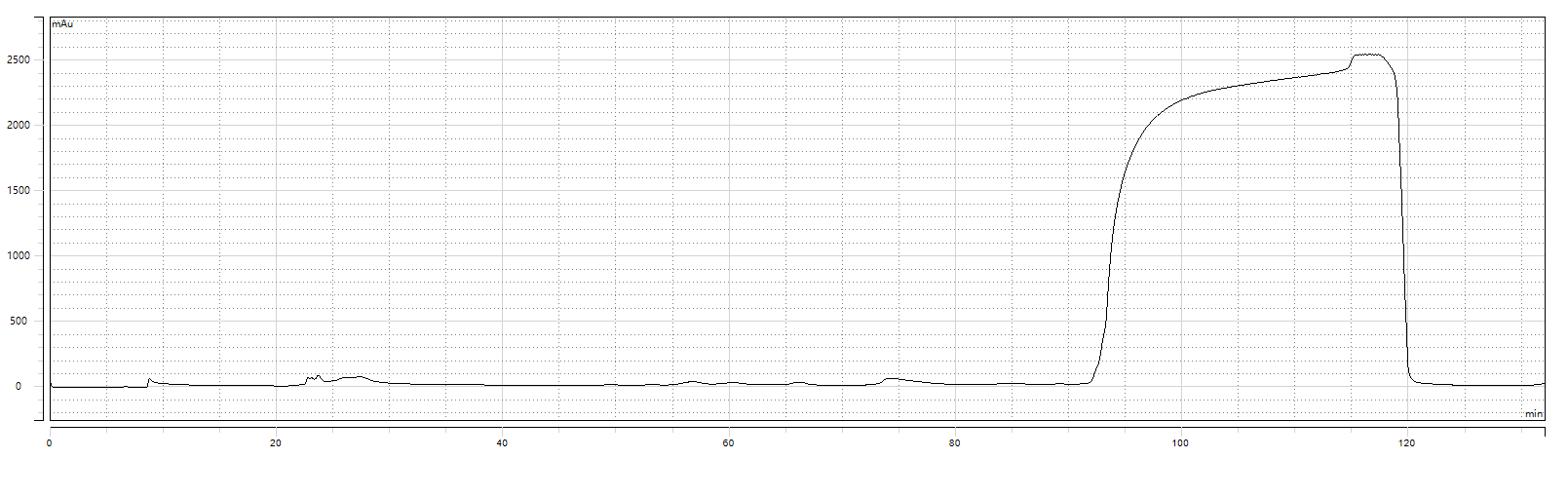
2nd Purification Test Results:Purity: 99.604%;Yield: 81.64%