Oligonucleotide drugs represent an innovative therapeutic approach with unique and potent mechanisms of action. These drugs regulate the expression of disease-causing genes at the fundamental level by interfering with gene transcription and translation processes, thereby achieving therapeutic effects. As one of the fastest-growing gene therapies, oligonucleotide drugs are typically composed of single-stranded or double-stranded nucleotides ranging from 12 to 30 units. They exert their effects by directly targeting disease-causing genes or acting on messenger RNA (mRNA) to modulate gene transcription and translation, offering high specificity. They are commonly synthesized via the solid-phase phosphoramidite chemistry method.
During the synthesis of oligonucleotide drugs, various side reactions can occur at multiple sites including the phosphate backbone, sugar ring, and bases. Additionally, factors such as synthesis efficiency and raw material quality lead to the presence of multiple impurities in crude products. One of the most challenging types of impurities is failed sequences (N-x/N+y) resulting from differences in single or multiple nucleic acid monomers. These N-x and N+y impurities exhibit very similar properties to the target product, with minimal differences in molecular size, charge, and hydrophobicity, posing significant challenges to subsequent purification processes. High-resolution purification technologies must be employed post-synthesis to separate the target molecules from impurities.
Challenges in Oligonucleotide Drug Purification Processes
Chromatographic steps are critical for controlling product-related impurities, and the core of chromatographic process development lies in mastering and utilizing the property differences between various impurities and the active pharmaceutical ingredient (API). Common property differences that may affect sample chromatographic behavior include the following:
Charged groups: The net charge of oligonucleotides depends on the number of phosphate or modified groups, the number of bases, and whether secondary structures shield charged groups. Hydrogen bonds are typically broken under denaturing conditions (e.g., pH 12) to unfold the structure and render bases uncharged (pH>pKa), enabling effective differentiation of N-1 impurities.
DMT on/off: DMT is a highly hydrophobic group that interacts with reverse-phase chromatography/hydrophobic interaction chromatography, making it useful for separating full-length sequences with DMT.
Thiolation: When oxygen atoms in phosphate groups are replaced by sulfur atoms, the polarity of negatively charged groups increases, leading to stronger binding with anion exchange resins under most pH conditions.
Methylation: Substitution of oxygen atoms with methyl groups enhances hydrophobicity.
Depurination: Excessive exposure to acid causes cleavage between purine bases and deoxyribose, altering molecular charge and hydrophobicity under certain pH conditions.
Therefore, chromatographic purification of oligonucleotide drugs primarily relies on differences in the number of charged and non-polar groups between API and process-related impurities (PRI). Depending on whether the final DMT group is retained after synthesis and whether on-column deprotection is selected, ion exchange chromatography or reverse-phase preparative chromatography is typically employed for purification.
1. Ion Exchange Chromatography
Anion exchange chromatography (AIEX) is a laboratory method for separating oligonucleotides under aqueous conditions. It eliminates the need for high-cost eluents containing organic solvents and explosion-proof design of workshops and equipment, making it particularly suitable for oligonucleotide purification. The most widely used resin is 15Q/30Q (HB-POR15Q from Hande Technology, a wholly-owned subsidiary of Hanbang Technology), with a polystyrene matrix, particle size of 15/30 μm, large operating range, and high loading capacity. It can still purify oligonucleotide samples under denaturing conditions (pH 12), avoiding aggregation of single-stranded self-complementary or GC-rich oligonucleotides.
Similar to protein purification, oligonucleotide purification processes need to consider multiple factors, such as purification buffer pH and salt ion concentration, loading capacity, flow rate, operating pressure, column bed height, and operating temperature. Elution salt concentration and volume must be adjusted based on whether the last DMT group is removed from the sample and the length of the oligonucleotide.
1.1 Purification Buffer pH and Salt Ion Concentration
Purification buffer pH and salt ion concentration are key factors for target molecule elution. A major application of high-pH eluents is to control hydrogen bond aggregation of single-stranded self-complementary or high-GC oligonucleotides. Most interactions can be disrupted at pH 12, but RNA is unstable under strongly alkaline conditions and must be purified in a near-neutral or weakly alkaline environment.
For subsequent industrial scale-up, the optimal high-salt elution ratio must be explored, preferably below 2M, with elution time not exceeding 0.5 hours. Excessively high halide ion concentrations can corrode stainless steel materials.
1.2 Sample Loading Capacity, Retention Time, and Column Packing Height
Oligonucleotides have low molecular weight and relatively few charged and hydrophobic groups. Retention time and loading capacity can be appropriately adjusted to achieve better separation results. For challenging projects, loading volume can be reduced to ensure purification purity, primarily because decreasing loading volume reduces both impurity and main peak areas while minimizing their overlap, facilitating higher single-tube purity during fraction collection.
Increasing column height also improves resolution. However, considering pressure issues with fine particle size resins and increased resin consumption (leading to higher costs) during industrial scale-up, the typical packing height ranges from 15 to 20 cm.
1.3 Purification Temperature
Purification temperature is a challenging parameter due to variations in thermal stability among different samples. Good purification results are generally achieved at room temperature, but optimization of purification temperature is sometimes necessary. Additionally, maintaining consistent temperature throughout the purification environment is crucial.
2. Reverse Phase Preparative Liquid Chromatography
Preparative Reverse Phase Liquid Chromatography (Prep-RPLC) is a technology that achieves separation and purification by leveraging the distribution differences of solutes between polar mobile phases and non-polar stationary phases, and accurately detecting and collecting corresponding fractions. In this technology, the stationary phase typically uses non-polar bonded phase resins, such as silica gel bonded with C18 or C8 alkyl chains, while the mobile phase consists of a mixture of polar organic solvents (e.g., methanol, acetonitrile) and water in a specific ratio.
RP-HPLC utilizes the hydrophobicity of the solid phase to effectively bind oligonucleotides with 5’-DMT. Nucleotide impurities without DMT protection are eluted first due to weaker binding, while oligonucleotides with 5’-DMT are eluted with stronger solvents, followed by removal of 5’-DMT to achieve effective separation.
2.1 Chromatographic Column Selection
High-pressure resistant and highly stable reverse-phase chromatographic columns (e.g., C18 resin) should be selected to withstand pressure fluctuations and long-term use in large-scale production, with a balance between resin loading capacity, resolution, and recovery rate as key considerations.
2.2 Mobile Phase Optimization
An acetonitrile-water system is typically used, with a small amount of acid (e.g., 0.1% formic acid) added to suppress interference from silanol groups on the silica gel surface and improve peak symmetry.
Ion-pairing reagents (e.g., triethylamine acetate, hexafluoroisopropanol) are added to the mobile phase, and mobile phase pH is controlled (e.g., 7.0-8.0) to stabilize ion-pair complexes. The hydrophobic end of the reagent binds to the oligonucleotide phosphate backbone, while the hydrophilic end dissolves in the aqueous phase, converting hydrophilic oligonucleotides into hydrophobic complexes and enhancing their retention on the reverse-phase chromatographic column.
2.3 Process Scale-Up Challenges
After laboratory-scale process development, linear scale-up to industrial scale is required, balancing factors such as loading volume, yield, purity, and cost.
Oligonucleotide Drug Purification Cases
Sample 1
Original sample purity: 75.4%
Purification column: LCC-10Column
volume: 19.63 ml
Packing material: Hedra Technology HB-POR15O
Linear velocity: 191.1 cm/h
Sample loading amount: 15.06 mg/ml
Collection range: UV 1260 nm, initial value 1000, end value 700
Mobile phase: Na₂HPO₄ (Phase A, ***M/L), Na₂HPO₄-NaCl (Phase B, ***M/L - ***M/L)
1.1 Purification method
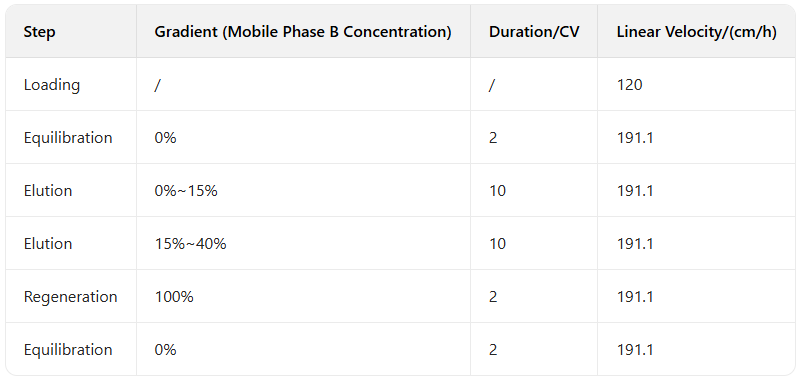
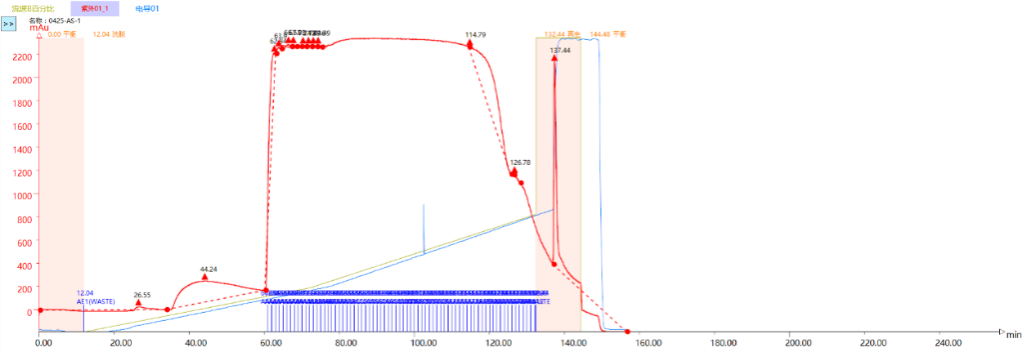
Chromatogram and Injection Results Summary
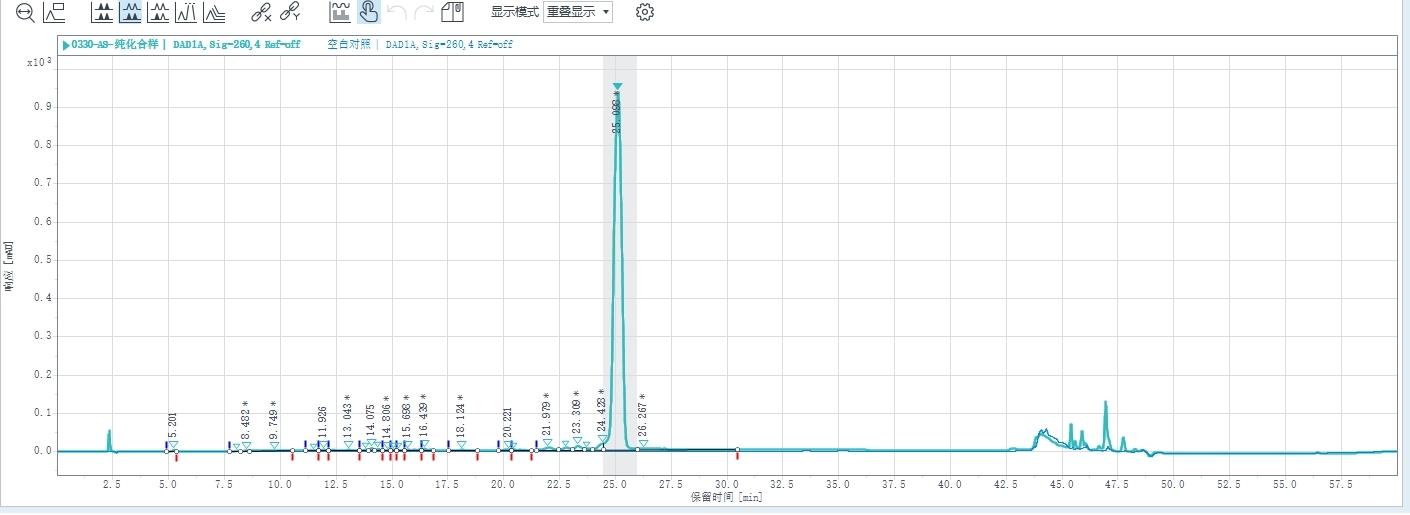
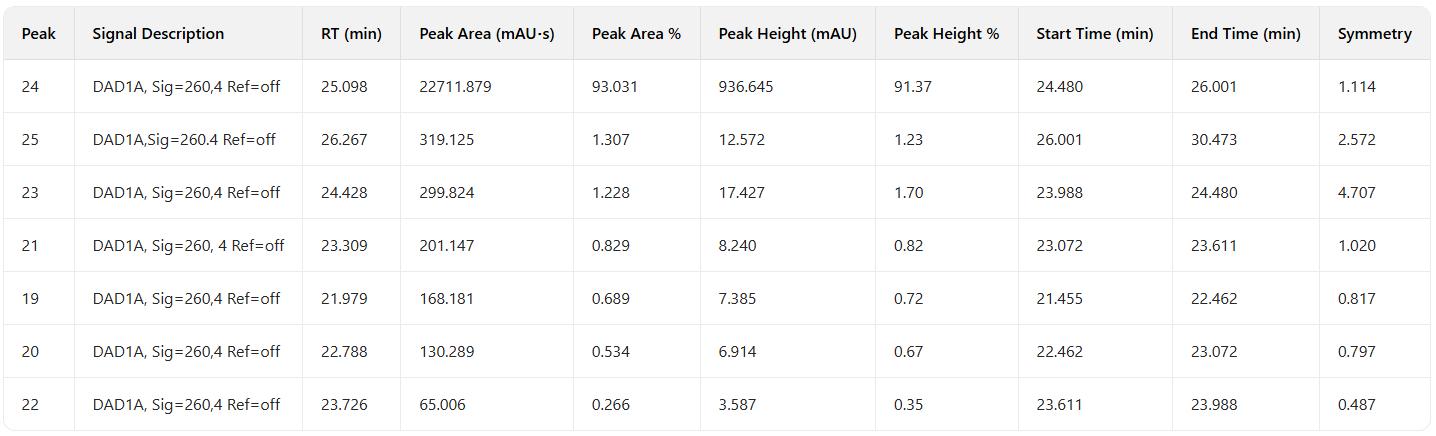
Combined sample purity: 93.031%, Sample yield: 81.3%
Sample 2
Raw sample purity: 86%
Column volume: 18.04ml
Loading amount: 13.13mg/ml
Linear velocity: 191cm/h
Purification column: LCC-10
Resin: Hedra Technology HB-POR15Q
Collection range: UV_1 260nm, start value 1000, end value 700
Mobile phase: Na₂HPO₄ (Phase A, ***mM/L), NaHPO₄-NaCl (Phase B, mM/LM/L)
Purification Method 1
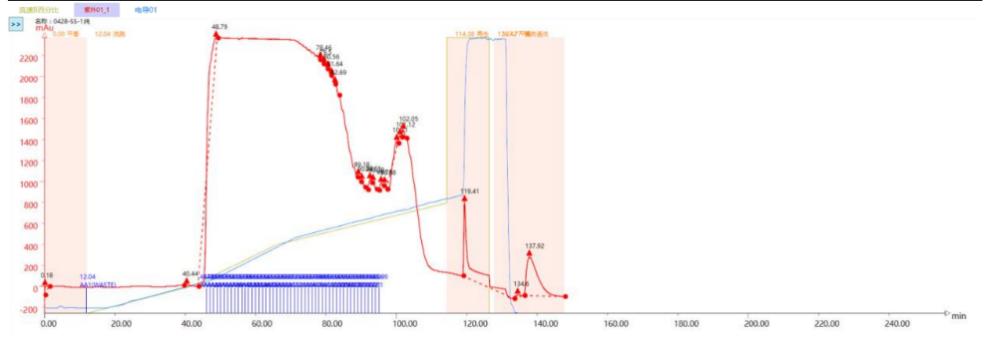
Chromatogram and Injection Results Summary
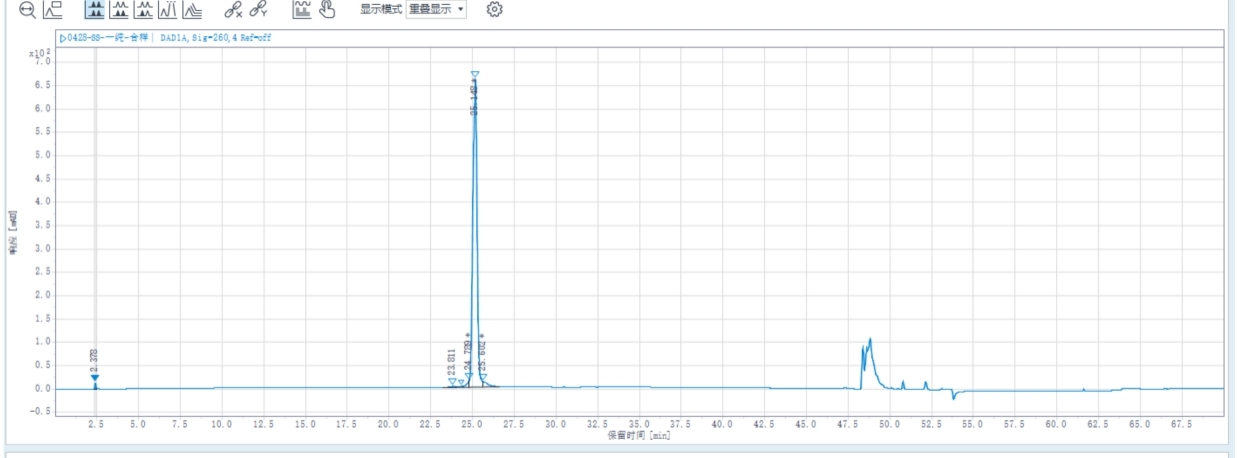
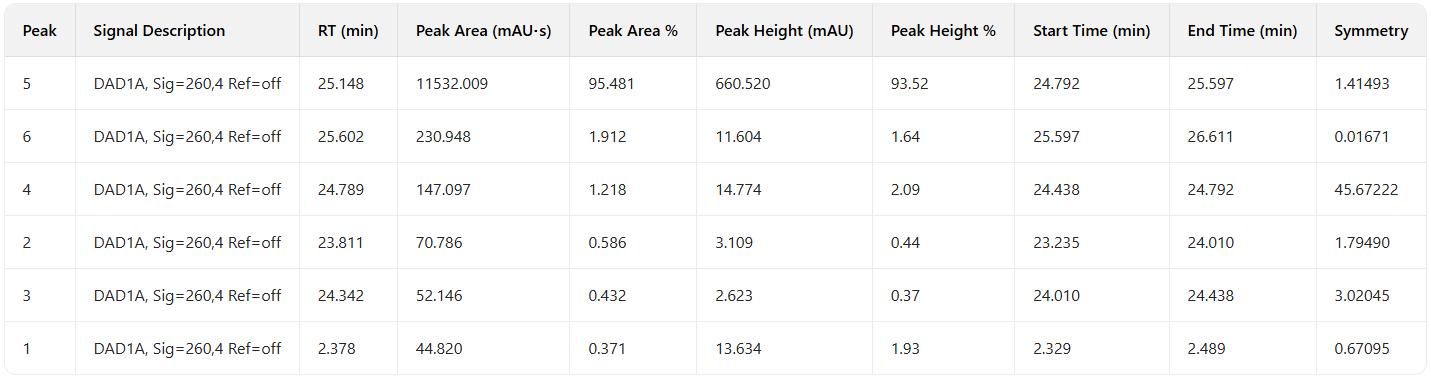
Combined sample purity: 95.48%, Purified sample yield: 75.74%
Sample 3:
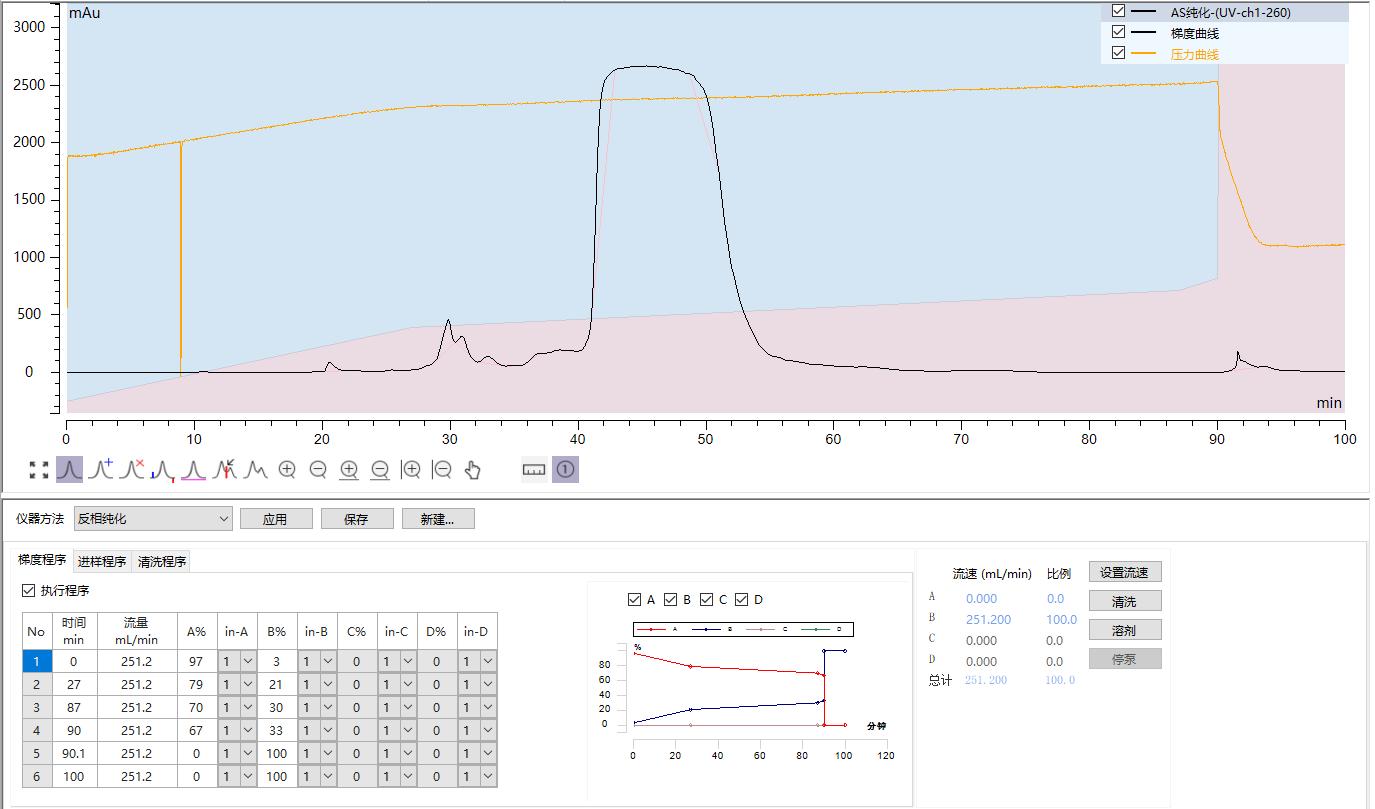
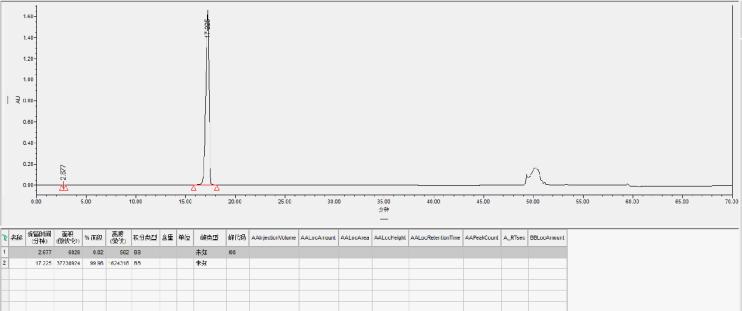
Inclisiran AS Strand Reverse Phase Purification
Preparation column packed with Hedra Technology HB-C18HAQ-100-30 resin
AS loading amount: 2.08mg/ml
Self-test purity: 99.98%
Yield: 95.01%
Sample 4: Inclisiran SS Strand Reverse Phase Purification
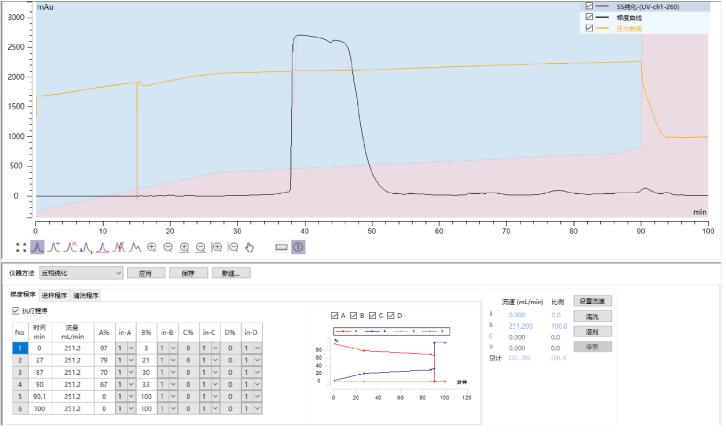
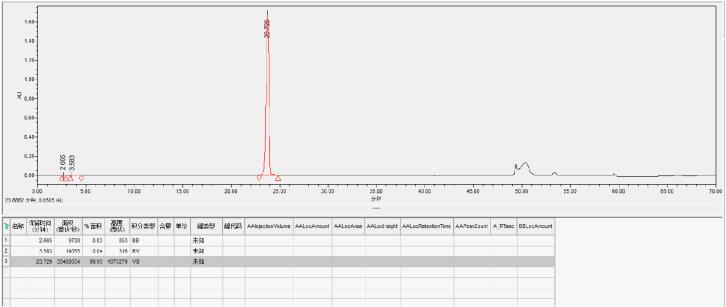
Preparation column packed with Hedra Technology HB-C18HAQ-100-30 resin
SS loading amount: 2mg/ml
Self-test purity: 99.93%
Yield: 90.9%
Hanbon Technology's Comprehensive Oligonucleotide Drug Solution
Hanbon Technology provides customers with a complete product portfolio covering nucleic acid synthesis systems, ion exchange chromatography systems, reverse phase preparative liquid chromatography systems, and tangential flow filtration systems, fully supporting all stages from basic research and process development to large-scale production. The company's product line meets the comprehensive needs of laboratory-scale exploration, pilot-scale process scale-up, and industrial-scale production. With stable and reliable system performance and flexible, scalable configuration solutions, it offers solid technical support and solutions for the entire lifecycle of small nucleic acid drugs from early R&D to commercial production.