A certain oligonucleotide bulk drug product requires filtration for desalination and bacterial endotoxin removal in the final production process. Desalination and bacterial endotoxin removal of materials is a critical procedure.
Three verification indicators were evaluated for tangential flow ultrafiltration (TFUF) technology: the endotoxin removal capability of the ultrafiltration system was confirmed by detecting bacterial endotoxin levels in the solution before and after filtration; the integrity of the ultrafiltration membrane module was tested post-use to ensure its structural soundness after filtration; and the Normalized Water Permeability (NWP) test was conducted to verify the cleaning efficacy of the membrane stack after each system use.
Verification Section
1. Preparation of Materials and Instruments
·•Tap water
·•Depyrogenated vials
·•Bacterial endotoxin test water
·•Bacterial endotoxin standard
·•50 mL graduated cylinder
·•1000 mL measuring cup
·•Tangential flow ultrafiltration system and membrane module
·•Endotoxin gel-clot assay detector
·•Clean bench
·•Vortex mixer
2. Experimental Methods
2.1 Bacterial Endotoxin Detection
For endotoxin control in small nucleic acid materials, control is primarily achieved through sterility and endotoxin management of the production environment, raw materials, and material-contacting equipment. Additionally, ultrafiltration with membrane modules of 30 kDa or 50 kDa molecular weight cut-off (MWCO) can effectively retain endotoxin polymers, allowing target small nucleic acid products to be collected at the permeate end and achieving partial endotoxin control. For buffer solutions, 6–10 kDa ultrafiltration membranes typically achieve >99.99% endotoxin retention efficiency.
Tap water was used as a surrogate for the pharmaceutical solution. Before and after ultrafiltration, 10 depyrogenated vials (3 mL per vial) of tap water were collected for bacterial endotoxin testing.
2.2 Acceptance Criteria
Bacterial endotoxin levels in tap water after ultrafiltration must be <0.25 EU/mL.
2.3 Experimental Results
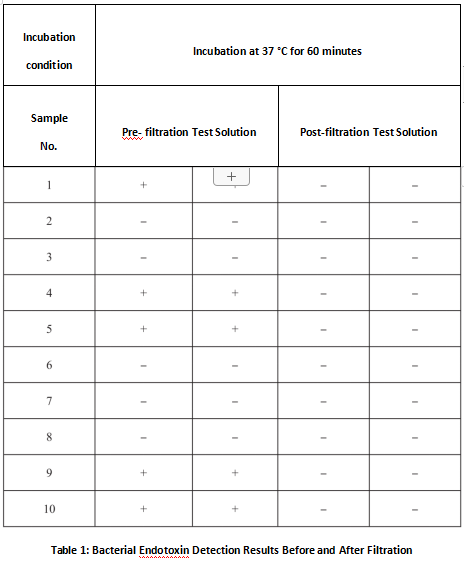
Test results indicated partial non-compliance of endotoxin levels in pre-filtration samples, while post-filtration samples met acceptance criteria, confirming the ultrafiltration system’s endotoxin removal capability.
2.4 Membrane Module Integrity Test
Membrane module integrity was verified using the Diffusion Flow Test (typical method), with the following procedure:
1.Self-check: Apply approximately 90 psi air pressure to the pipeline via a proportional control valve; monitor pressure changes at P1, P2, and P3 to detect leaks.
2.Pipeline evacuation: Apply approximately 15 psi air pressure to purge the pipeline of impurities, ensuring it is filled with clean compressed air.
3.Upstream volume calculation: Fill an air tank (volume v₁) with compressed air at pressure p₁ via a proportional valve; switch solenoid valves to fill the upstream space (volume v₂) with tank air, then measure upstream pressure p₂. Calculate v₂ using the closed-space gas displacement formula:p₁v₁ = p₂v₂
4.Diffusion flow rate calculation: Based on the ideal gas law (m = PV/RT), fill the tank with compressed air, release it to the membrane, and calculate gas loss volume per unit time (diffusion flow rate) from pressure changes.Diffusion flow rate formula:D = ΔP × V / (Pt100 × t)
·ΔP: Pressure decay during measurement
·V: Upstream volume of the filter element/membrane
·Pt100: Standard atmospheric pressure
·t: Measurement time
5.Completion: Open solenoid valve V5 to release compressed air from the pipeline.
The Bio-TFF Test Integrity Tester is an automated membrane integrity device with four built-in test modes: pressure hold, bubble point, diffusion flow, and immersion. It features fast detection, simple operation, customizable test parameters (by membrane type), automatic data logging (audit trail compliant), and test result printing.
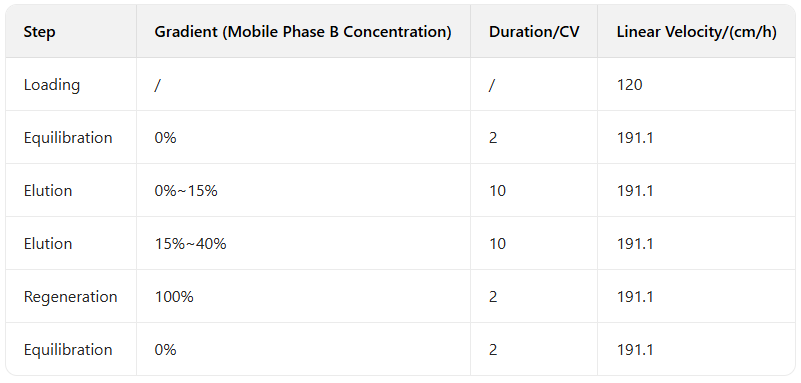
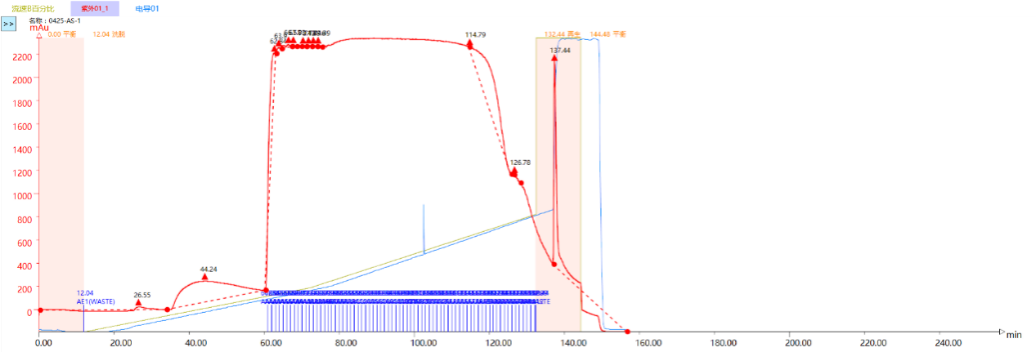
Table 2: System Parameters of Bio-TFF Test Integrity Tester
2.5 Normalized Water Permeability (NWP) Test
NWP testing measures membrane flux under controlled conditions; normalized NWP values reflect membrane performance (typically ≥80% of the initial baseline).
Test Procedure:
1.Set the TFUF system to full recirculation mode (retentate and permeate ends connected to the feed vessel).
2.Fill the circulation tank with sufficient deionized water/water for injection; fully open feed, retentate, and permeate valves.
3.Start the feed pump; adjust pump speed and retentate valve to achieve a transmembrane pressure (TMP) of 0.33 bar (5 psi). Calculate permeate flow rate from permeate weight (balance) and time; record feed water temperature.
4.Adjust pump speed/retentate valve to achieve 1 bar (15 psi) TMP; repeat flow rate/temperature measurement.
5.Drain the system.
6.Calculate NWP using the formula:
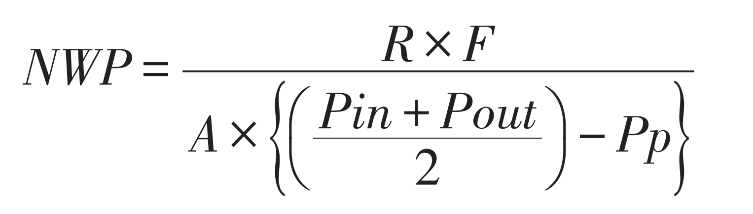
-R: Permeate rate (mL/min)
·F: Temperature correction factor
·A: Membrane area (m²)
·Pu: Inlet pressure (bar)
·Pp: Permeate pressure (bar)
7.Plot NWP vs. TMP.
8.Correct NWP for temperature (viscosity): e.g., 115 LMH at 0.7 bar (10 psi) and 18 °C → 115 LMH × 1.053 = 121 LMH (normalized to 20 °C).
9.Convert to standard NWP (1 psi TMP): Divide by 10 psi (e.g., 121 LMH ÷ 10 = 12.1 LMH/psi).
Hanhuang Technology’s ultrafiltration system features built-in automated NWP testing for membrane modules.
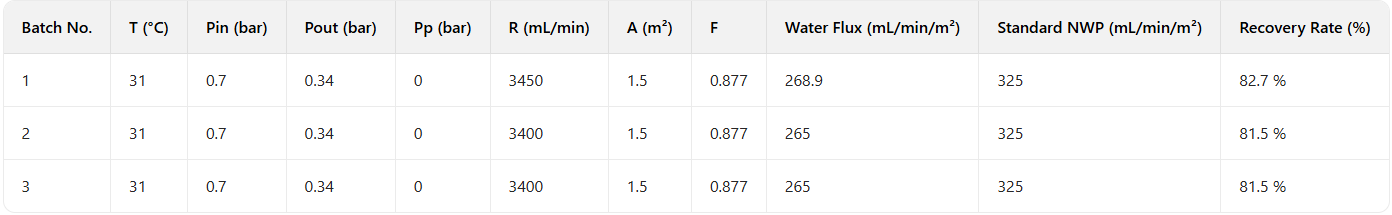
Table 3: NWP Test Results
Verification data confirmed NWP recovery rates >80%, indicating minimal NWP attenuation after repeated membrane use. This validates the suitability of cleaning agents/procedures for routine production.
3. Concentration and Desalination Procedures
3.1 Membrane Pore Size Selection
Membranes are selected by small nucleic acid molecular weight (e.g., 1–3 kDa MWCO for 5–30 nt oligonucleotides). Two membrane materials are available: polyethersulfone (PES) or regenerated cellulose (RC).
3.2 Operating Steps
·Concentration: Load purified small nucleic acid solution into the ultrafiltration system; concentrate to target volume (e.g., 10% of initial volume) via tangential flow recirculation (TMP: 1.0–1.5 bar) to avoid membrane fouling from over-concentration. Small membrane pore sizes result in low permeate flux (even at high TMP), making optimal concentration ratio/liquid exchange timing critical for process efficiency.
·Desalination: Replace the stock solution with low-ionic-strength buffer (e.g., TE buffer or pure water) 3–5 times until conductivity meets specifications.
Taking the concentration of the AS strand of siRNA drugs (inclisiran) as an example, after concentration and desalination, the sample concentration showed no loss, and the recovery rate could reach over 96%。
4. Conclusion
Verification of the tangential flow ultrafiltration system and membrane modules confirmed suitability for oligonucleotide concentration, desalination, and endotoxin removal. Routine cleaning procedures ensure effective membrane cleaning, validating the technical protocol for daily production use. This verification reduces maintenance costs and supports stable system operation.